Search by application
Search by method of connection with device
Select by device/piping connection type
-
Search Products
-
Industrial Hoses
-
Search by application
-
Search by usage conditions
-
Select from chemical resistance information
-
Search by model
-
-
Industrial Couplings
-
Search by method of connection with device
-
Search by method of connection with hose
-
Search by usage conditions
-
Select from compatible couplings for your hose
-
Select from chemical resistance information
-
-
KAMLOK
-
Search by product category
-
Select by device/piping connection type
-
Search by usage conditions
-
Select from chemical resistance information
-
-
Gardening/watering products
-
Simplified piping system
-
-
Support and Downloads
- Inquire Now
- Hose Adviser Pro
Phone:+81-765-52-3131
- TOP
- ・
- Toyox Support
- ・
- List of Product-Related Documents
- ・
- FDA Registered Products
FDA Registered Products

[ About FDA-Compliant and Registered Hoses ]
To ensure safe and reliable use, TOYOX offers a lineup of FDA-compliant and registered hoses, including silicone hoses, tetrafluororesin hoses, and PVC food-grade hoses (excluding some products).
Click here for the list of FDA-compliant and registered hoses >>>
TOYOSILICONE Hose Series products (hoses, assemblies, integrally molded couplings) are registered in the Drug Master File (DMF) Type II as of January 2012.TOYOFUSSO Hose Series products (hoses, assemblies) are registered in the Drug Master File (DMF) Type II as of May 2018.
The registration number is 25486.
*However, safety is not guaranteed in its entirety.
*It is necessary for customers to judge the particular safety conditions of their respective workplaces beforehand. (Precautionary reference material)
A list of registered companies’ DMF files is available on the FDA’s official site here>>>
(The number on the left, “25486,” is the listing for the TOYOSILICONE Hose and TOYOFUSSO Hose Series products)
As proof of FDA certification, Toyox hose and coupling products bear the below markings:
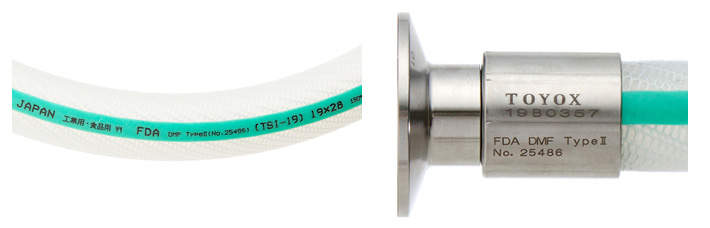
*DMF register number markings are on products made from April 2012, however, products manufactured before that time that do not have the marking are also treated as registered products.
About the FDA
- The FDA, the U.S. Food and Drug Administration, is similar to the Japanese Ministry of Health, Labor and Welfare, and is one part of the HHS(Department of Health and Human Services). It is an abbreviation for Food and Drug Administration.
- The purpose of the FDA is to provide market approval, carry out inspections both before and after distribution, and review the safety and efficacy of medicine and food related products in order to ensure the safety of American citizens.
FDA examination standards are very strict and known as proof of a products safety and reliability.
Notes for use
TOYOSILICONE Hose Series products (Hoses, assemblies and integrally molded couplings), and TOYOFUSSO Hose Series products (hoses and assemblies), are developed and produced for general industry applications.
For applications that require safety, confirm in advance.
Never use for implant or injection application or other applications where there is a possibility of the product partially remaining in the body.
Toyox makes no guarantee of the adaptability or safeness related to such applications.